Are you searching for 'write a lewis structure for each molecule or ion cn'? You will find questions and answers on the subject here.
Table of contents
- Write a lewis structure for each molecule or ion cn in 2021
- Cn-1 lewis structure
- Cn- lewis structure formal charge
- Cn- lewis structure molecular geometry
- How to draw the best lewis structure
- Cn- structure
- Formal charge of cn-
- Write a lewis structure for each molecule or ion cn 08
Write a lewis structure for each molecule or ion cn in 2021
 This image demonstrates write a lewis structure for each molecule or ion cn.
This image demonstrates write a lewis structure for each molecule or ion cn.
Cn-1 lewis structure
 This picture demonstrates Cn-1 lewis structure.
This picture demonstrates Cn-1 lewis structure.
Cn- lewis structure formal charge
 This image demonstrates Cn- lewis structure formal charge.
This image demonstrates Cn- lewis structure formal charge.
Cn- lewis structure molecular geometry
 This picture demonstrates Cn- lewis structure molecular geometry.
This picture demonstrates Cn- lewis structure molecular geometry.
How to draw the best lewis structure
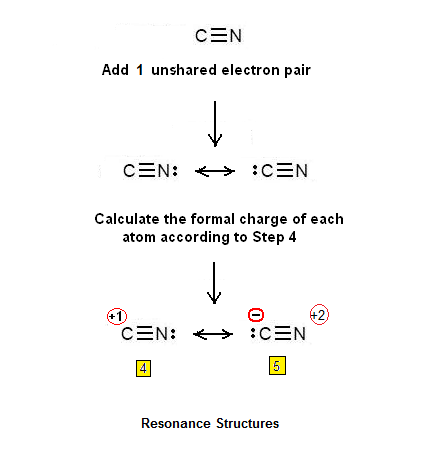 This picture demonstrates How to draw the best lewis structure.
This picture demonstrates How to draw the best lewis structure.
Cn- structure
 This picture representes Cn- structure.
This picture representes Cn- structure.
Formal charge of cn-
 This image illustrates Formal charge of cn-.
This image illustrates Formal charge of cn-.
Write a lewis structure for each molecule or ion cn 08
 This picture illustrates Write a lewis structure for each molecule or ion cn 08.
This picture illustrates Write a lewis structure for each molecule or ion cn 08.
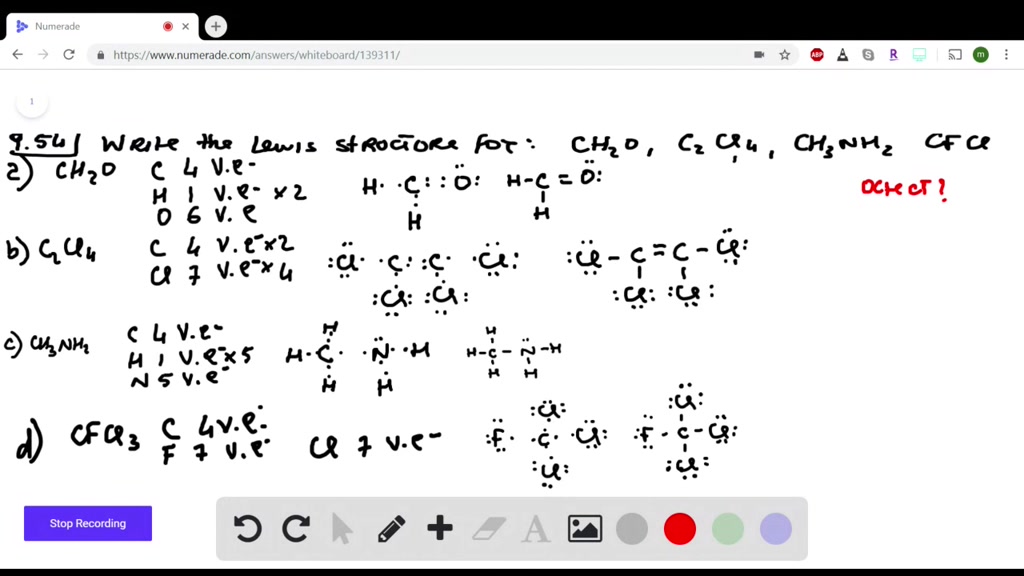
How many valence electrons are in the CN Lewis structure?
The Lewis structure for CN- has a total of ten valence electrons. In order to make sure the outer shell of the Nitrogen atoms are full you will need to form a triple bond in this Lewis structure (between the Nitrogen and Carbon atoms).
How to draw the dot structure for CN-Lewis?
For the CN- Lewis structure, calculate the total number of valence electrons for the CN- molecule. After determining how many valence electrons there are in CN-, place them around the central atom to complete the octets. The Lewis structure for CN- has a total of ten valence electrons.
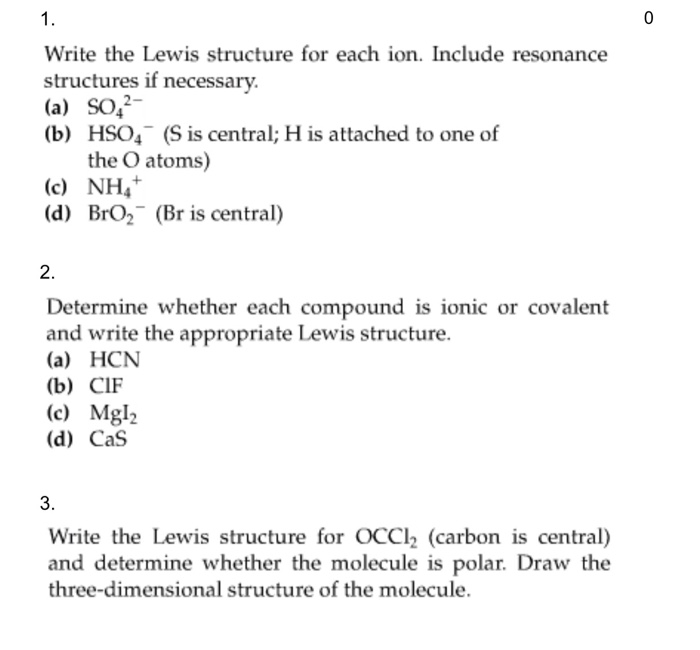
How to determine the Lewis structure of a molecule?
To determine the Lewis structure of any given molecule, it is vital to first know the total number of valence electrons for the molecule.
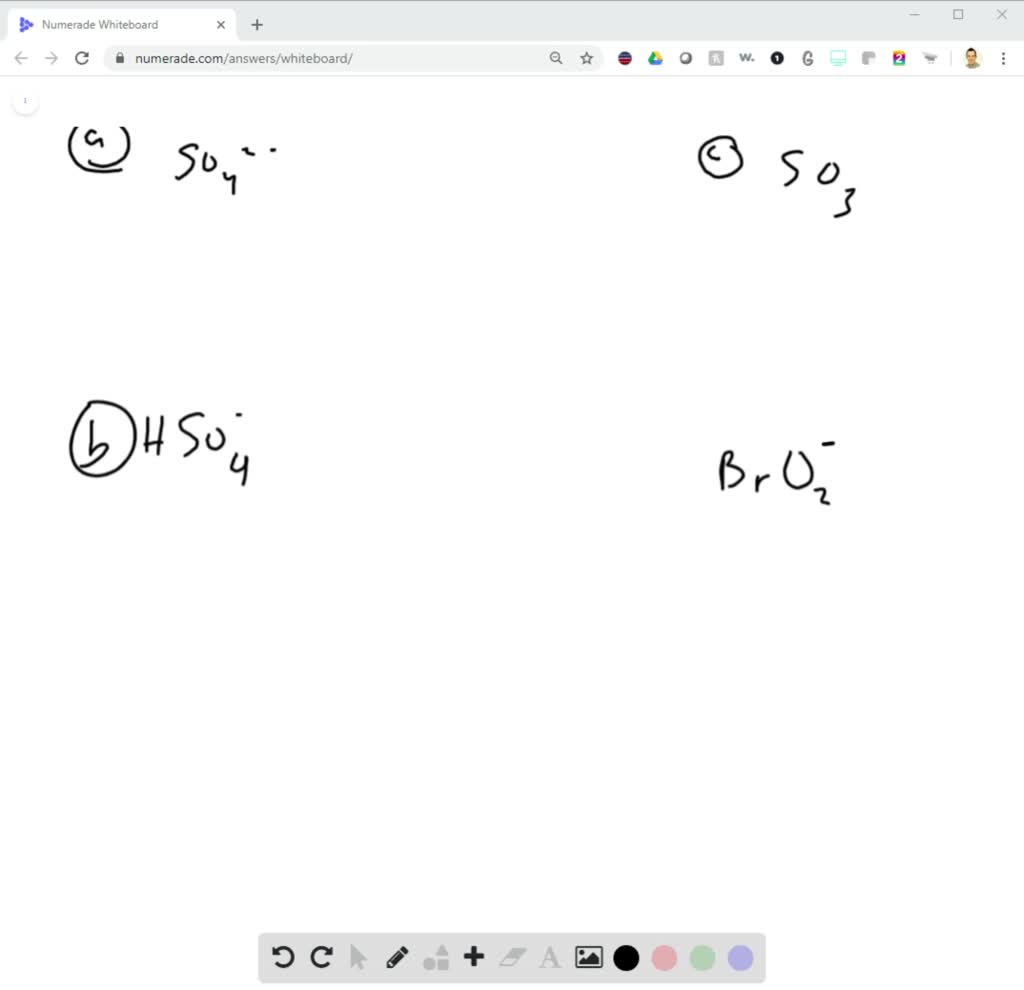
How is the structure of a CN-molecule determined?
CN- has quite a simple structure to understand. It is a linear molecule, and both the atoms are arranged as far as possible to minimize the repulsive forces of the lone pairs of electrons present on both these atoms. Hence, it has a linear shape. CN- polar or nonpolar
Last Update: Oct 2021
Leave a reply
Comments
Lavinia
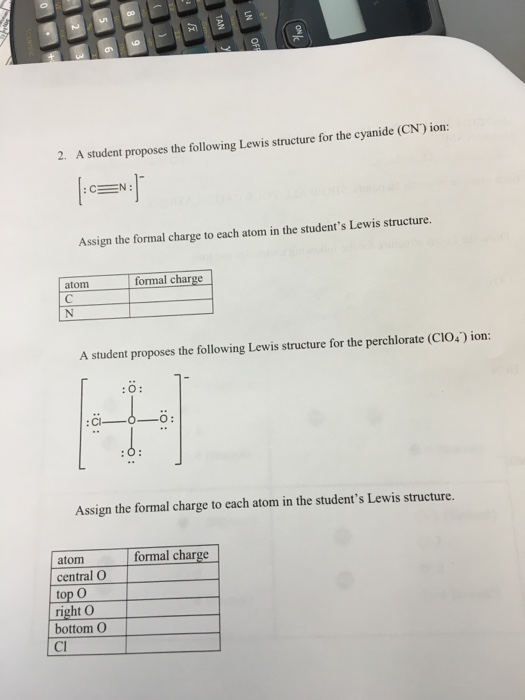
28.10.2021 11:33Hydrogencarbonate is the atomic number 6 oxoanion resulting from the removal of a proton from carbonic acid. Assign titular charges to complete atoms.
Aviana
24.10.2021 04:11Jerry Lee Lewis structure helps stylish deriving the geometry of the molecules. Application: use the mote cards and cheerios to build the molecule before draft it.
Brita
21.10.2021 03:43Pen the lewis body structure for each ion. The central atom of this molecule is carbon.